How to Perform Dynamic Multicolor Time-Lapse Imaging
Fast Live-Cell Imaging
Challenges
Fast intracellular events can be challenging for live-cell microscopy in terms of the following technical limitation. The temporal correlation of dynamic multicolor time-lapse imaging relies on the capability of the imaging system to record different fluorescent channels at the same time. Imagine a plane with one red and one green light flying in the sky. If your imaging system is only capable of recording one of the lights (red OR green) at a time, the plane will move forward in between the two consecutive images. Consequently, in a time-lapse movie you will not be able to tell if this is one (a red- and green-light plane), or two different planes (one red-light or one green-light plane).
Fast intracellular events can be challenging for live-cell microscopy in terms of the following technical limitation. The temporal correlation of dynamic multicolor time-lapse imaging relies on the capability of the imaging system to record different fluorescent channels at the same time. Imagine a plane with one red and one green light flying in the sky. If your imaging system is only capable of recording one of the lights (red OR green) at a time, the plane will move forward in between the two consecutive images. Consequently, in a time-lapse movie you will not be able to tell if this is one (a red- and green-light plane), or two different planes (one red-light or one green-light plane).
Transferring this limitation to multicolor time-lapse imaging means, that you would not be able to properly correlate signals of two different fluorescence signals, e.g., a cargo protein and a motor protein of a fast-moving vesicle. This artifact is known as a spatiotemporal mismatch and is often an inherent problem of sequential imaging.
Classical fluorescence filter-based imaging systems suffer from the technical limitation described above, because changing filter cubes for the different fluorophores takes time.
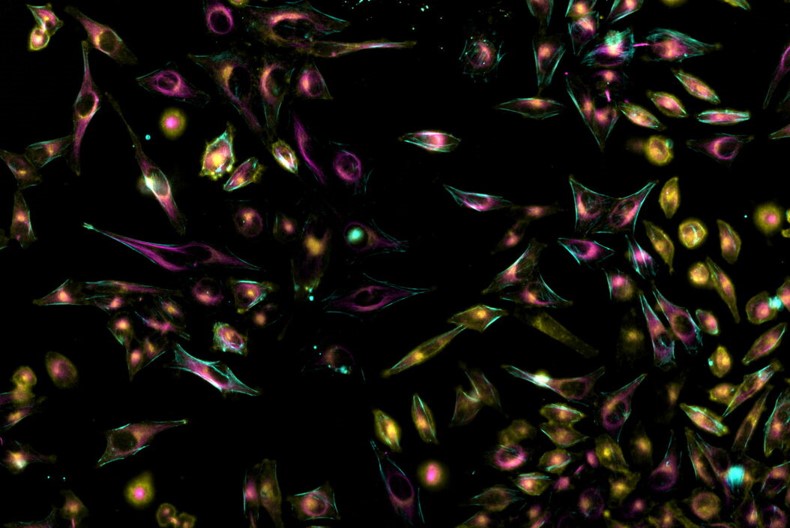
Mica with its FluoSync™ technology (Figure 1) can acquire up to four fluorescence channels simultaneously. With it, 100% spatiotemporal correlation of four different cellular elements can be achieved. Additionally, exposure times of single channels do not add up with a simultaneous approach. Thus, the overall time consumption to acquire one time-point of a time-lapse experiment is shortened compared to sequential acquisition. Furthermore, Mica is an incubator which keeps cells at a given temperature and CO2 level over long time periods to have near-physiological imaging conditions.

Figure 1: FluoSync™ detection hardware with fully integrated digital spectral hybrid unmixing for the acquisition of up to 4 labels simultaneously. FluoSync™ captures photons across the visible spectrum, discarding less information than narrow bandwidth filters, and then separates each signal by employing a phasor-based hybrid unmixing approach, which results in robust channel separation.
Methods
Living cells were cultured either in Petri dishes or 96-well plates. For live-cell imaging, Mica was operated at 37°C, 5% CO2, and high humidity (to avoid concentration increases of solvents due to water evaporation). Imaging parameters are mentioned in the paragraphs below.
Results
In the following paragraphs, there are a few examples of live-cell imaging with Mica. Starting with an example showing the advantage of simultaneous imaging, over widefield THUNDER acquisition, through to high-speed confocal imaging.
1. Simultaneous vs. Sequential Imaging in CLSM mode
Videos 1a) and 1b) show the difference between sequential and simultaneous imaging. The same vesicle population was stained with two different live-cell dyes (green and red), meaning that all vesicles should appear yellow – the overlay of green and red.
Video 1a) shows both channels taken sequentially, one after the other. In this case, several vesicles appear to be either red or green, although they are both. It can be extremely difficult to differentiate red or green stationary vesicles from double stained moving ones.
In the Video 1b), both channels were acquired simultaneously. Here, all vesicles appear yellow due to the overlay of red and green.
Video 1a): Objective HC PL
APO
CS2 63x/1.20 WATER. Cells were stained with WGA-Alexa Fluor™ 488 (green)) and WGA-Alexa Fluor™ 555 (magenta) at the same time. Video shows both fluorophores acquired sequentially. Several vesicles appear as two distinct vesicles, one green and one magenta vesicle.
Video 1b): Objective HC PL
APO
CS2 63x/1.20 WATER. Cells were stained with WGA-Alexa Fluor™ 488 (green)) and WGA-Alexa Fluor™ 555 (magenta) at the same time. Video shows both fluorophores acquired simultaneously. All vesicles show a colocalization of both WGA dyes and mostly appear white.
2. Color Time-Lapse Imaging - Widefield vs. THUNDER
In Video 2, HeLa cells were stained with WGA-Alexa Fluor™ 488 and SiR-Tubulin. The single images of the 3D time-lapse were taken every 5 seconds in widefield mode for a total duration of 2 minutes. Total z-stack size was 1.1 µm consisting of 6 layers.
The appropriate THUNDER method can be started and then synchronized with image acquisition or executed post acquisition.
Video 2: HeLa cells stained with WGA-488 (yellow) and SiR-Tubulin (magenta). Objective HC PL
APO
CS2 63x/1.20 WATER. Simultaneous acquisition, 100 ms exposure time. 3D time-lapse imaging with 5-second intervals for a total duration of 2 minutes.
Top: Widefield. Middle: Instant Computational Clearing. Bottom: Large Volume Computational Clearing.
3. Color Time-Lapse Imaging - Widefield vs. THUNDER
Video 3: HeLa cells stained with WGA-488 (yellow), SPY-Actin (cyan), and SiR-Tubulin (magenta). Objective is HC PL APO CS2 63x/1.20 WATER. Simultaneous acquisition with 100-ms exposure time. Time-lapse imaging with 9second intervals for 2 minutes duration. Maximum projection of a 9-layer z-stack of 1.8 µm total size. Top: Widefield. Bottom: Large Volume Computational Clearing.
4. Color Time-Lapse Imaging – CLSM
Video 4: U2OS cells stained with MitoTracker™ Blue (blue), FluoView 488 Tubulin (green), WGA-Alexa555 (yellow), and SiR-Actin (cyan). Objective is HC PL APO CS2 63x/1.20 WATER. Simultaneous acquisition. Time-lapse imaging with 5-second intervals for a total duration of 2 minutes.
5. Color Time-Lapse Imaging – CLSM – High Speed
Video 5: U343-tfLC3 cells stably expressing Tandem-LC3 ( GFP (cyan)-mCherry (yellow)) and additionally stained with MitoTracker™ Deep Red (magenta) and NucBlue™ (blue). Objective is HC PL APO CS2 63x/1.20 WATER. Time-lapse data acquired over a total of 1 minute and with 4 channels simultaneously in widefield mode with a time interval of 200 ms between each time point. Left: Widefield RAW, Right: THUNDER Instant Computational Clearing.
Conclusions
Mica enables users to acquire the signal of up to four fluorophores at a time, either in confocal or widefield mode, including LIGHTNING and THUNDER. With it, cellular components can be imaged at the very same point in time, without spatiotemporal mismatch.
Nenechte si ujít další zajímavosti
- Vanquish Access – dostupné řešení spolehlivých HPLC analýz
- Psychoreologie a tribo-reologická charakterizace pudinků ve vztahu k textuře
- Adsorbovatelné organické fluoridy (AOF): Klíč k modernímu monitoringu PFAS ve vodě
- Elementární rozbor whisky pomocí icp-ms: detailní pohled do stopového světa oblíbeného destilátu
- Thermo Scientific Cindion C-IC (Combustion Ion Chromatography) - komplexní řešení pro stanovení celkových halogenů a síry ve složitých matricích